This simulation is computationally intensive. Be sure not to leave the simulation running in the background of other applications as you may experience a decrease in your computer's performance.
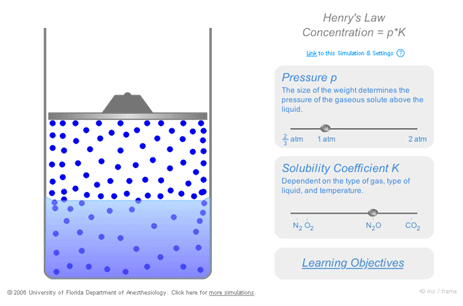
You must be a registered instructor to access this simulation. Instructor registration is $100/year and your privacy will be respected. If you are not already an instructor, click here. In this transparent reality simulation of gas solubility, the concentration of gas molecule icons above the liquid represents the concentration of gas in the gas pocket and is proportional to its partial pressure. The concentration of gas molecule icons in the liquid is proportional to the concentration of gas dissolved in the liquid. Tension of the dissolved gas is not explicitly depicted in the transparent reality simulation. However, tension can be inferred from the concentration of gas molecule icons in the gas pocket because tension is equal to the partial pressure of gas above the liquid. The ratio of the concentration of dissolved gas to the partial pressure of gas above the liquid is the solubility coefficient. This transparent reality simulation illustrates arguments for consistently using the term “tension” instead of |
|
“partial pressure” to denote the pressure exerted by a dissolved gas. The magnitude of the weight represents ambient pressure. The simulation represents a closed volume; the total number of gas molecule icons in the simulation (whether in the gas pocket above the liquid or dissolved in the liquid) is constant. A companion Flash paper document explains the simulation (in English) and is accessed by clicking on the “Learning Objectives” link. Partial pressure in the gas phase and the solubility coefficient K are user adjustable via their respective “click and drag” slider bars. User can subsequently visualize how Henry’s Law governs the resulting changes for gases that do not react with the liquids in which they dissolve. | |